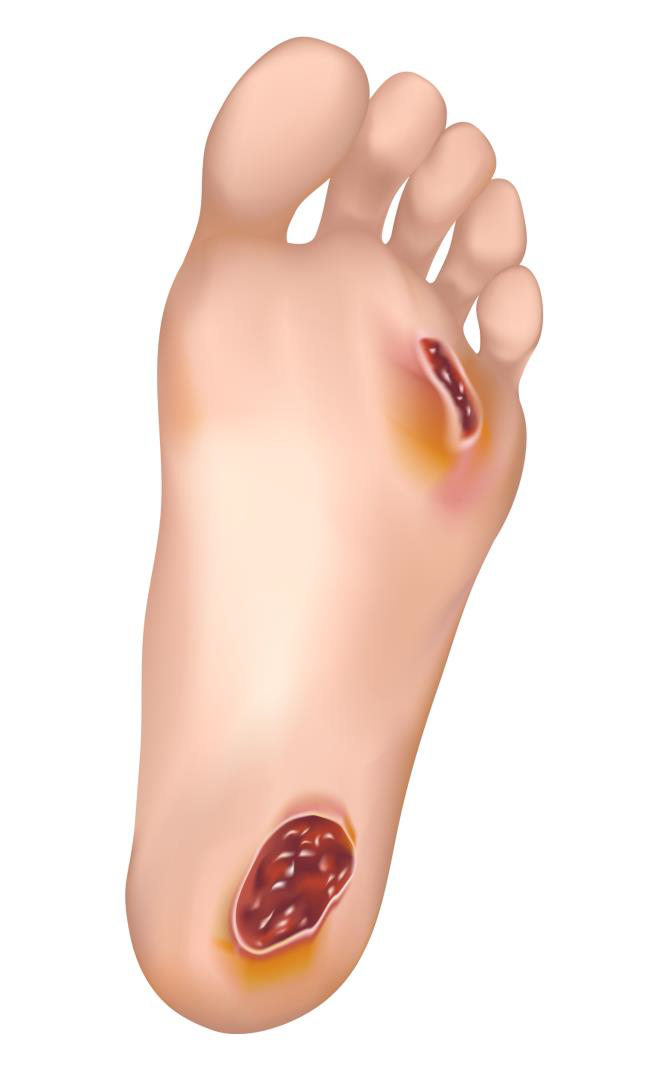
Description
This herbal medication can heal all types of wounds, including old, infectious, diabetic, and bedsores. It can rebuild destroyed tissues and is highly effective in treating burns.
Clinical tests have demonstrated its effectiveness in treating 1st,2nd, and 3rd-degree burns, as well as scratches, deep cuts, eczema, and psoriasis.
The difference and properties of powder and ointment compared to other burn medicines
1- After using the medicine, the burning sensation disappears within 5 minutes.
2- It is applied once on the wound and does not need to be redressed.
3- The duration of treatment is as follows:
– Superficial wounds: 1 to 2 days
– Semi-deep wounds: 1 to 7 days
– Deep wounds: 7 to 19 days
This duration is unique globally even compared to chemical drugs.
4- Both medicines are entirely herbal.
5- After the treatment, there are no scars, and the skin looks the same as before, even with hair growing in its original places.
6- It is unparalleled in treating burns, wounds, and infections.
The global market for the treatment of wounds and skin injuries
Based on statistics from around the world, it is evident that wound treatment generates significant financial turnover.
However, conventional methods are often less effective than our product or end up being quite costly.
This also holds true for the treatment of skin injuries, albeit with a lower turnover.
Wound Healing Methods
The lack of a strong product to improve wound healing methods results in a variety of approaches and extended treatment time. This product has the potential to replace many traditional methods.
1-Collagen for Wound Healing
2-Keratinocytes
3-Biological dressings (BD)
These methods either fail to deliver the same results as our product or are very expensive.
4-Biological skin equivalents (BSE)
5-Platelet-derived growth factor (PDGF)
6-Silver products
7-Negative pressure wound therapy (NPWT)
8-Hyperbaric oxygen (HBOT)
9-Topical oxygen
10-Ozone oxygen
11-Herbal material
Advantages of our product over the Chinese products:
This product uses powder instead of ointment, allowing oxygen to reach the wound.
For the entire treatment period, it is used only for onetime,except for grade 3 wounds with significant depth.
There is no need to change the dressing, and the effective substance on the wound is not lost. This is unlike the Chinese product, which needs to be used twice a day.
Repairing skin injuries and burns,unlike the Chinese product, which is used only for wound treatment.
Report on the evaluation of the healing effect on burn wounds:
Explanation of laboratory results
To confirm the effect of the pharmaceutical granular product “Pardis Powder” on the healing of surface burns, related animal tests were conducted.
The method, measured variables, and the obtained results are announced as follows.
Method of analysis:
Rats weighing 300 ± 20 grams were selected to perform this test.
Trichotomy:
Trichotomy was performed on the skin on the back of the neck of the animals, and in order to heal the resulting inflammation, one day was considered before the burn.
Creating a standard burn:
Laboratory animals were anesthetized by ketamine-xylosin cocktail.
The back of the neck of the rats was disinfected with betadine and then cleaned with 70% ethanol.
An aluminum probe with a weight of 51 grams and a diameter of 1 cm remained in boiling water at 100 degrees Celsius for 10 minutes.
After drying the aluminum probe, it was immediately placed vertically on the animal’s skin for 15 seconds.
Skin application of the product:
Following the burn, laboratory animals were subjected to treatment with the specified products over a 30-day period. The animals were categorized into three groups: control (C), test (T), and positive
control (+). Subsequent to moistening the wounds with sterile gauze and normal saline, they were
covered with normal saline, Pardis Powder, and alpha ointment, correspondingly.
Immediately after the burn on day 0, the surface of the wound was photographed by a professional
high-precision camera. This was repeated every day after cleaning the wound and before taking the
medicine. The obtained images were used to check tissue changes and measure the healing rate of the
wound surface with the help of Image Tools software.
Results:
- Crust tissue is a dark and dry layer that has a strong connection to the underlying inflamed tissue of the wound.
This layer is created by the migration of platelets and the formation of fibrin layers on the surface of the wound.
According to qualitative studies, “Pardis Powder” leads to the acceleration of the formation of crust tissue on the surface of the burn wound. The comparison of different experimental groups showed that in the group receiving this product, the formation of this layer on the wound surface was completed 2-3 days faster than the control group and 1-2 days faster than the alpha ointment group.
- The rate of wound surface reduction (wound contraction) is the percentage of wound surface area reduction over time, which is a measure of wound surface repair. According to the obtained data, “Pardis Powder” did not show any difference in the rate of wound surface reduction compared to the control group and the alpha ointment group. Relevant data is included in the attached file.
- Scar tissue left over from wound healing is the result of new collagen formation at the wound site. The extent of scar tissue depends on the severity and depth of the burn so that scar tissue does not form in superficial first-degree burns.
Comparing the wounds treated with “Pardis Powder” with the control group showed that the use of this product can probably lead to a decrease in the level of scar tissue left from seconddegree burn wounds.
Conclusion:
It seems that because of the high probability of infection of burn wounds by bacteria, such as Pseudomonas, it is one of the potential risks of burn wounds. It seems that the local use of
“Pardis Powder” reduces the risk of secondary infection due to the acceleration of crust formation on the burn wound.
On the other hand, due to the properties of “Pardis Powder” granular product in absorbing secretions and superficial dead tissue of the burn wound, this product probably leads to limiting the final scar caused by the wound by reducing the accumulation of inflammatory cells in the burn bed.
Determining the Soluble and Insoluble Ash Content in Powder:
Two grams of the samples (plant leaves and processed plants) were weighed and placed in a
pre-weighed crucible, then subjected to a 600-degree Celsius oven for complete calcification.
Upon cooling the crucible, the total ash content was calculated using a scale accurate to 0.0001
grams.
Subsequently, the total ash was dissolved in 25 ml of 10% hydrochloric acid and heated to boiling. After cooling, the samples were filtered and dried in a 100-degree incubator,followed by further heating in a 600-degree oven until the filter paper completely disappeared.
After cooling the samples in a desiccator, they were re-weighed using a scale accurate to 0.0001 grams to calculate the amount of ash insoluble in acid and its percentage.
Powder Original plant powder
14.06 7.9 % total ash
1.3 2.3 % ash insoluble in acid
The qualitative assessment of Marham ointment
Qualitative assessment of the thin-layer chromatography method
Determining the Soluble and Insoluble Ash Content in Powder Two grams of the samples (plant leaves and processed plants) were weighed and placed in a pre-weighed crucible, then subjected to a 600-degree Celsius oven for complete calcification.
Upon cooling the crucible, the total ash content was calculated using a scale accurate to 0.0001 grams.
Subsequently, the total ash was dissolved in 25 ml of 10% hydrochloric acid and heated to boiling. After cooling, the samples were filtered and dried in a 100-degree incubator,followed by further heating in a 600-degree oven until the filter paper completely disappeared.
Determining the moisture amount in burn powder:
The moisture content of the provided sample was determined using the weight loss method within a 100-degree Celsius incubator.
A 2-gram portion of the sample was placed in a pre-weighed crucible and subjected to the 100-degree incubator. The sample was periodically weighed, using a scale with 0.0001 accuracy, at 10-minute intervals until the observed weight changes exceeded 0.05 grams.
The moisture content of the sample: 0.042 grams.
Assessing the total phenolic content of the provided product using the Folin-Ciocalteu method with a Spectrophotometer:
Initially, 2 grams of plant powder will be extracted before and after the burning process. This will involve immersing the powder in a waterethanol solvent (50%) for 2 hours using a 90-degree bain-marie. Subsequently,the solution will be subjected to filtration. The resulting solution will then undergo drying on a 90-degree bain-marie and subsequent dissolution in hot water to establish 1.5 mg/ml concentrations from both samples.
Subsequently, the absorbance of each tannic acid sample was gauged at a 765 nm wavelength using a spectrophotometer. This process was reiterated thrice for each tannic acid dilution, and the absorbance data were used to construct the tannic acid calibration curve.
In this investigation, the Folin-Ciocalteu solution functioned as the reagent, and tannic acid served as the standard phenolic compound for the analyses. Initially, one milliliter of tannic acid solution at various dilutions (25, 50, 100 micrograms per milliliter) was combined with 5 milliliters of
Folin-Ciocalteu reagent, diluted at a ratio of 1:10, and left to incubate at room temperature. Following a 10-minute interval, 4 milliliters of sodium carbonate solution (7.5 mg/liter) were introduced.
The resultant mixture was then subjected to a 30-minute incubation period at room temperature, shielded from light.
A similar procedure was executed for each extracted sample, with the exception that 1 milliliter of extract with a concentration (μg/ml) was utilized instead of tannic acid.
Determining the amount of total phenolic content of the extract based on the tannic acid standard curve:
| Extract hydroalcoholic | Concentration(mg/ml) | Total phenolic/tannic | Total phenol % |
| Original plant powder | 0.75 | 127.87 | 17.05 |
| Burn powder (Gaz) | 1.5 | 82.37 | 5.49 |
Qualitative assessment of the thin-layer chromatography method
1.Two grams of the ointment were dissolved in 20 ml of methanol and its solubility was enhanced using acetone. This solution underwent sonication at 40 degrees Celsius for 15 minutes and was subsequently filtered for further utilization.
- The stationary phase utilized was silica gel GF manufactured by Merck (Art: 5554), complemented by solvents for the mobile phase sourced from the same company
- Thin-layer chromatography sheets, each measuring 10 x 3,were prepared.
- Each sheet was marked at the 0.5cm range from the end using a pencil.
- The spotting place was accurately determined.
- Sampling included 10 μl from each plant sample and approximately 20 μl from its derived product.
- Each TLC from step 6 was placed in the chromatography tank in accordance with the suitable solvent designated for the plant sample.
- Subsequent to passing the toluene-ethyl acetate (93:7) mobile phase solvent over the TLC stationary phase, the sheets were removed from the chromatography tank and air-dried in the laboratory.
- Evaluation of the TLCs upon their emergence from the chromatography tank was initially conducted using a UV lamp. Subsequently, each TLC was subjected to reagent smearing using vanillin in sulfuric acid with the aid of a reagent spray tool.
- Determination of standards for thin-layer chromatography was based on the product’s components.